Also known generically as a Reconstructed Human Epidermis (RHE), EpiDerm is a ready-to-use, highly differentiated 3D tissue model consisting of normal, human-derived epidermal keratinocytes (NHEK) cultured on specially prepared tissue culture inserts.

Cultured at the air-liquid interface (ALI), EpiDerm allows for the evaluation of topically applied compounds, chemicals, cosmetic/personal care product ingredients and final formulations. With our straightforward protocols and 20+ years of data, EpiDerm is the gold standard for a broad range of highly predictive in vitro applications.
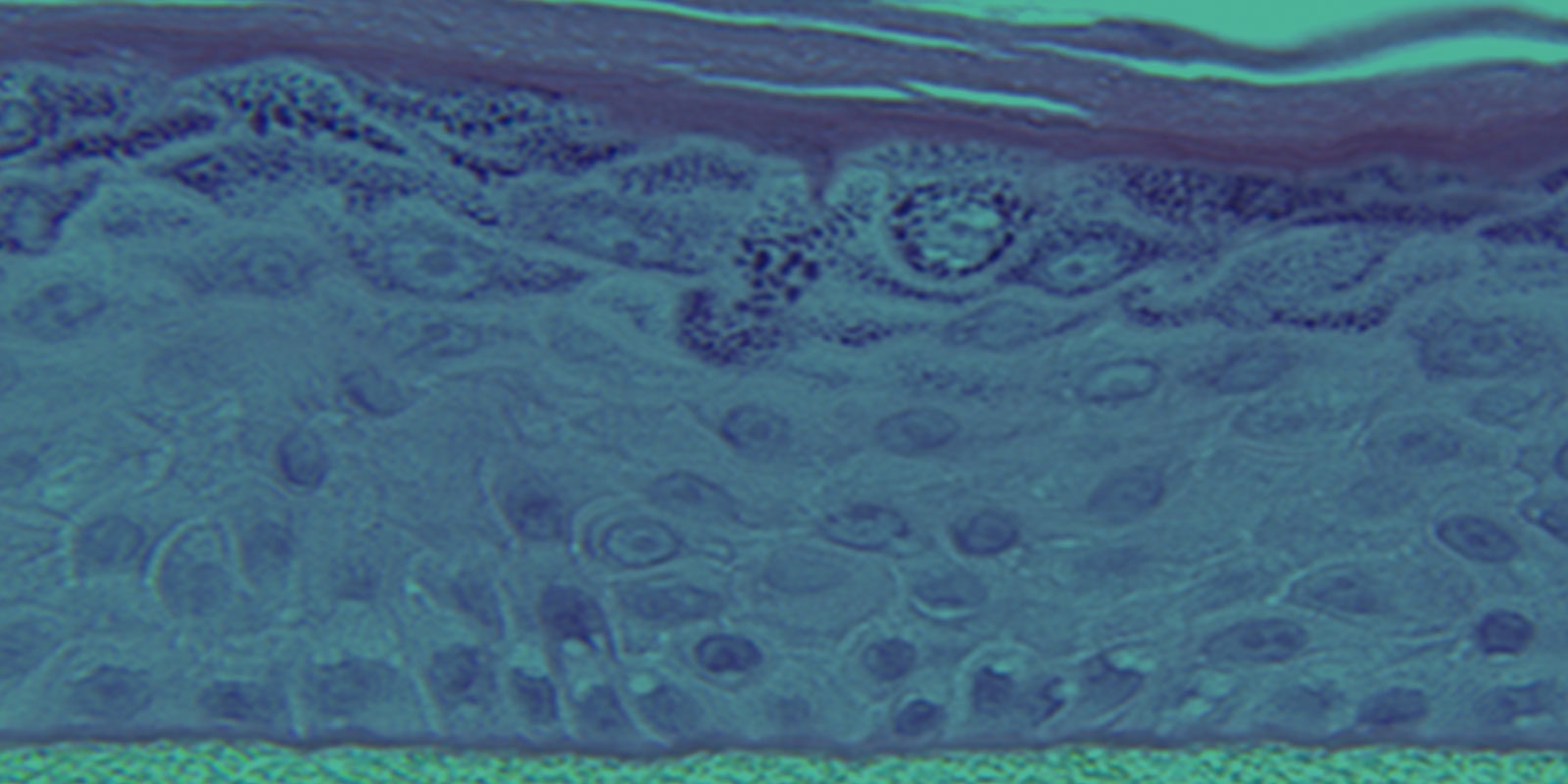
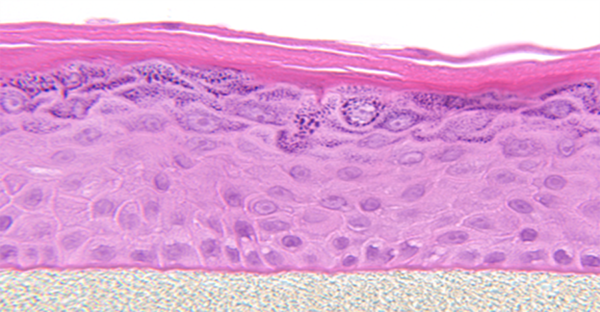
EpiDerm exhibits human epidermal tissue structure and cellular morphology with greater uniformity and reproducibility. It’s 3D structure consisting of organized and proliferative basal cells, spinous and granular layers, and cornified epidermal layers are mitotically and metabolically active.
The EpiDerm 3D human tissue model is used across a diverse range of applications including safety and risk assessment, and biological efficacy. With Air-liquid Interface (ALI) culture technology, EpiDerm can be used to evaluate biological responses to topical applications of formulations or ingredients. Simple protocols and the evaluation of early cellular endpoints allow research to acquire data in days, not weeks or months.
Skin Irritation (OECD TG 439)
Use EpiDerm to determine the skin irritation potential of liquids, solids, gels, lotions, ointments, or creams. Tissue viability results are acquired within 3 days.
Download the Skin Irritation Test Protocol
Skin Corrosion (OECD TG 431)
Determine the skin corrosion potential of test materials in a single day.
Download the Skin Corrosion Protocol
Skin Hydration
Measure the electrical impedance of EpiDerm to assess the efficacy of topically applied moisturizers including creams, gels, and lotions. The Skin Hydration protocol allows for data analysis within 24 hours.
Skin Hydration Application Note
Dermal Drug Delivery
Utilize existing transdermal permeation equipment or MatTek’s simple single insert permeation devices to assess API permeation and flux. Determine relative safety with the EpiDerm MTT ET-50 assay.
Download the Percutaneous Absorption Protocol
Phototoxicity (OECD TG 498)
Identify phototoxic effects of topically applied test substances using the EpiDerm Phototoxicity Assay.
Download the Phototoxicity Protocol
Identify the effects of systemically applied test substances using the EpiDerm tissue model.
Download the Systemic Phototoxicity Protocol
Dermal Genotoxicity
Identify genotoxic effects of topical or systemic test substances using EpiDerm Genotoxicity Assays (pre-validated)
Comet Assay Protocol
Epidermal Differentiation
Evaluate active compounds for effects on epidermal differentiation using the under-developed EpiDerm tissue model
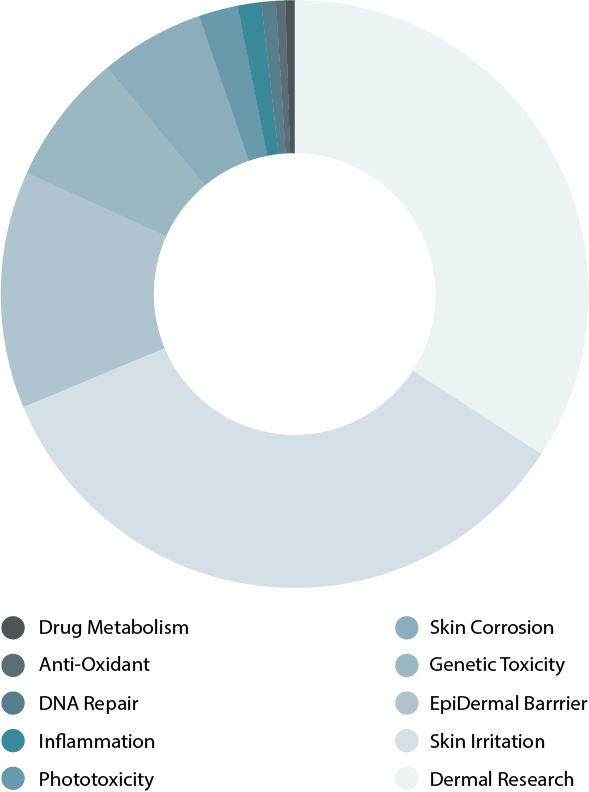
Browse our reference library to see how our EpiDerm tissue has been used in these areas of study.
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**