Get the latest
Get MatTek offers and updates delivered to your inbox.
During product development of new personal care or cosmetic formulations, the eye irritation potential is evaluated to identify which may cause adverse corneal reaction. Understanding and evaluating the irritation potential of chemicals or formulations is an important consideration to avoid exposing human volunteers to potentially irritating materials during clinical testing.
The in vitro EpiOcular MTT ET-50 assay is used as a screen to assign expected in vivo irritancy responses based on the time to toxicity (ET-50) results obtained with EpiOcular. This test is also used to rank order test formulations according to their eye irritation potential.
Protocol
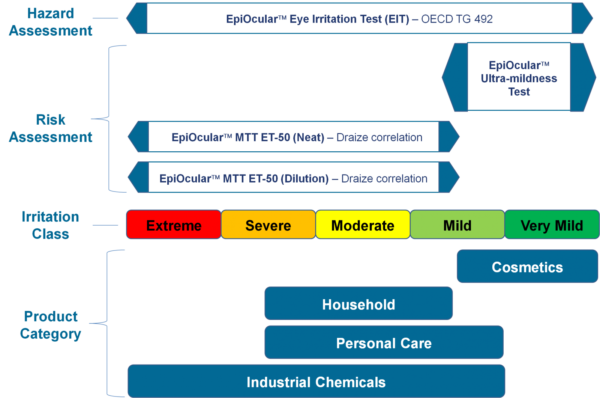
EpiOcular “Sub-Draize” Ultra-mildness Test
The EpiOcular Ultra-mildness Test is an in vitro risk assessment assay routinely utilized for mild materials for which the Draize test is insensitive. This highly reproducible assay allows for quantifiable discrimination among mild, milder and mildest product formulations.
Download the EpiOcular MTT ET-50 Sub-Draize Protocol
EpiOcular MTT ET-50 (Neat)
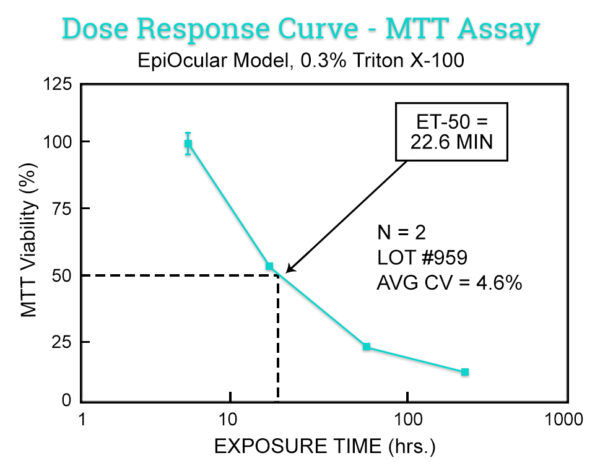
This in vitro risk assessment assay is based on the effective time at which a material (applied neat) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS).
Download the EpiOcular MTT ET-50 Neat Method Protocol
EpiOcular MTT ET-50 (Dilution)
Similar to the MTT ET-50 Neat method, this in vitro risk assessment assay is based on the effective time at which a material (diluted to 20% in water) causes a 50% reduction in tissue viability (ET-50). Based on the ET-50, the test article is categorized into one of 4 classifications ranging from non-irritating to severe/extreme which correspond to groupings of Rabbit Draize Eye Scores (MMAS). This method is recommended for surfactant-based solutions and is applicable to water-soluble materials with a specific gravity of > 0.95.
Download the EpiOcular MTT ET-50 Dilution Method Protocol
Data
References
Kandarova, H. et al., (2018). CON4EI: EpiOcular Eye Irritation Test (EpiOcular EIT) for hazard identification and labeling of eye irritating chemicals. Toxicol. in Vitro 49, 21-23.
Kandarova, H., Klausner, M., Kubilus, J., Ayehunie, S., Hayden, P., Kaluzhny, Y., Letasiova, S., and Sheasgreen, J. Update on Validation Status and Industry Utilization of Normal Human 3D NHu-3D Animal Alternative Models. Presented at 8th World Congress on Alternative and Animal Use, Montreal, Canada, 2011.
Klausner, M., Osborn, M., Bellavance, K., Breyfogle, B., Kubilus, J., Cerven, D.R., and DeGeorge, G. The EpiOcular Prediction Model: in vivo Versus in vitro Draize Scores for Consumer Products. Presented at the 2000 Society of Toxicology Meeting.
Kubilis, J., Sennott, H., Ogle, P., and Klausner, M. Reproducibility and Correlation of EpiOcular, a Three-Dimensional Tissue Culture Model of the Human Corneal Epithelium. Presented at the 1996 Society of Toxicology Meeting.
Request a Quote
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.