The EpiDerm™ Skin Irritation Test for Medical Device Extracts is designed to predict the skin irritancy potential of extracts from medical devices according to the requirements of ISO standards 10993-23:2021. It is used to evaluate medical device extracts that may contain irritants at very low concentrations, and allows for the discrimination between irritants and non-irritants, and is a reliable, ethical, and biologically relevant validated assay to assess the dermal irritation potential of medical devices.
The results of a validation study conducted in 2016 and 2017 by 17 laboratories around the world and several other scientific papers related to this project are now published in Toxicology In Vitro in a special issue dedicated to Medical Devices. Based on the results of this project, the Working Group 8 for Irritation and Sensitization of the ISO TC 194 on Biological and Clinical Evaluation of Medical Devices proposed a working draft of ISO guideline 10993-23 for Determination of Skin Irritation of Medical Device Extracts using RhE models.
Protocol
| Test Model | EpiDerm |
| Replicates | N=3 tissues per extract |
| Exposure Conditions | 18 hours topical exposure to 100µl of each extract |
| Test Article Quantity | 1mL or 1g |
| Assay Controls | Negative Control – sterile DPBS Vehicle Control 1 – saline (0.9% NaCl in ultrapure H2O Vehicle Control 2 – sesame oil Positive Control 1 – 1% SDS in saline (v/v) Positive Control 2 – 1% SDS in sesame oil (v/v) |
| Test Material | Extracts from each test material are prepared in saline as well as in sesame oil |
| Extraction Condition | 37 ± 1 °C for 72 ± 2 hours with continuous agitation/shaking |
| Endpoints | MTT Viability Assay |
| Data Analysis | % relative viability ± SD In vitro classification |
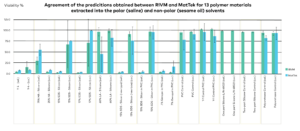
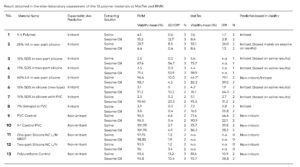
Data
Click images to enlarge.
Reference: Helena Kandarova, Jamin A. Willoughby, Wim H. De Jong, Silvia Letasiova, Tatiana Milasova, Michael A. Bachelor, Bridget Breyfogle, Yuki Handa, Liset De la Fonteyne, Kelly P. Coleman. Pre-validation of an in vitro skin irritation test for medical devices using the reconstructed human tissue model EpiDerm™, Toxicology in Vitro, Volume 50, 2018, Pages 407-417, ISSN 0887-2333, https://doi.org/10.1016/j.tiv.2018.02.007.
References
United Nations (UN) (2007). Globally Harmonized System of Classification and Labeling of Chemicals (GHS), Second revised edition, UN New York and Geneva, 2007. Available: [http://www.unece.org/trans/danger/publi/ghs/ghs_rev02/02files_e.html]
OECD, 2002. OECD Guideline for Testing of Chemicals, No. 404: Acute Dermal Irritation/Corrosion. Organisation for Economic Cooperation and Development, Paris.
Kandárová H, Willoughby JA, De Jong WD, Letasiova S, Milasova T, Bachelor MA, Breyfogle B, Handa Y, De la Fonteyne L, Coleman KP. (2018) Pre-validation of an in vitro skin irritation test for medical devices using the reconstructed human tissue model EpiDerm™. Toxicolog Vitr 50: 407-417. doi: 10.1016/ j.tiv.2018.02.007
Kandárová H, Bendova H, Letasiova S, Coleman KP, De Jong WD, Jirova D. (2018) Evaluation of the medical devices benchmark materials in the controlled human patch testing and in the RhE in vitro skin irritation protocol. Toxicolog Vitr 50: 433-438. doi: 10.1016/ j.tiv.2018.02.009
De Jong et al (2018). Round robin study to evaluate the reconstructed human epidermis (RhE) model as an in vitro skin irritation test for detection of irritant activity in medical device extracts, 2018. Toxicol In Vit 50: 439-449. https://doi.org/10.1016/j.tiv.2018.01.001
International Standard, ISO 10993-23:2021, Biological evaluation of medical devices — Part 23: Tests for irritation (https://www.iso.org/standard/74151.html).
Request a Quote
Thank you for requesting information about Mattek products! A representative will contact you shortly.
**If you would like to place an order for Mattek products, please contact Customer Service**