Get the latest
Get MatTek offers and updates delivered to your inbox.
The EpiDerm Phototoxicity Assay measures acute toxic response after exposure of skin to certain chemicals and subsequent exposure to light or that is induced similarly by skin irradiation after systemic administration of a chemical substance. The test is based upon a comparison of the cytotoxicity of a chemical when tested with and without additional exposure to a nontoxic dose of UVA + visible light and is accepted by the OECD as a stand-alone in vitro phototoxicity test.
Protocol
| Test Model | EpiDerm |
| Replicates | N=2 tissues per test condition |
| Exposure Time | O/N exposure to 50 µl of test article diluted in oil or H2O per tissue, followed by +/- exposure to 6J/cm2 of UVA |
| Assay Controls | Vehicle Control – Sesame Oil/H2O Positive Control – Chlorpromazine dose range |
| Endpoints | MTT Tissue Viability Assay |
| Data Delivery | % Viability relative to control |
Download the EpiDerm Phototoxicity Protocol
Download the EpiDerm Systemic Phototoxicity Protocol
Data
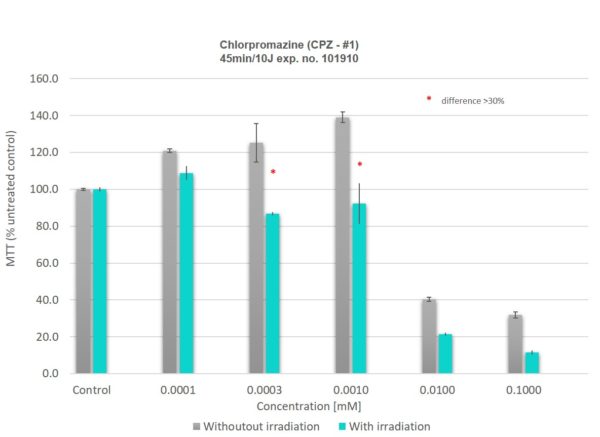
Results in as little as 72 hours.
References
Kandarova H., Liebsch M. (2017) The EpiDerm™ Phototoxicity Test (EpiDerm™ H3D-PT). In: Eskes C., van Vliet E., Maibach H. (eds) Alternatives for Dermal Toxicity Testing. Springer, Cham.
Request a Quote
Get MatTek offers and updates delivered to your inbox.