Changing the Future of Women’s Health

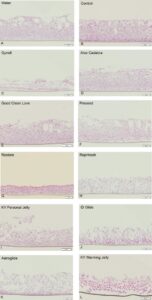 analyzed after 24 hours for tissue viability and permeability. The results confirmed that the OTC lubricants showing greater than 4 times (and some up to 30 times) the osmolality of in vivo vaginal fluid damaged human vaginal tissue barrier integrity. This is significant because this damage has been shown to increase the risk of infections, such as bacterial vaginosis (BV) whose estimated global cost is over $4.5 billion a year. Furthermore, BV can lead to more serious health conditions, from premature births to sexually transmitted infections like HIV, the virus that causes AIDS. “If products on the market were required to pass the animal-free tests we employed, many of them would be taken off the shelves,” Strgar points out.
analyzed after 24 hours for tissue viability and permeability. The results confirmed that the OTC lubricants showing greater than 4 times (and some up to 30 times) the osmolality of in vivo vaginal fluid damaged human vaginal tissue barrier integrity. This is significant because this damage has been shown to increase the risk of infections, such as bacterial vaginosis (BV) whose estimated global cost is over $4.5 billion a year. Furthermore, BV can lead to more serious health conditions, from premature births to sexually transmitted infections like HIV, the virus that causes AIDS. “If products on the market were required to pass the animal-free tests we employed, many of them would be taken off the shelves,” Strgar points out.
When it comes to personal lubricants for women, the research with the EpiVaginal showed that existing animal testing did not offer the complete picture, especially because the vaginal tissue of rabbits and guinea pigs do not respond in the same way to hyperosmolal formulations. The EpiVaginal model more accurately mimics the physiology of women and represents, as Strgar puts it, “an in vivo model without actually having to put a woman through that.” In the end, Strgar’s passion, Dr. Cone’s research and Mattek’s model were instrumental in convincing the FDA to accept nonanimal models, such as the EpiVaginal, as alternatives for the RVI test.
Beyond animal testing
 The support and expertise from Mattek helped Good Clean Love, a certified B Corp committed to balancing profit and purpose, diversify and expand its portfolio of personal lubricants, feminine hygiene and vaginal care products. Strgar’s persistence proves that anyone can influence and inspire leaders to change the future of health. “I feel proud that we’ve taken these steps with the FDA on behalf of better women’s health products,” Strgar says. “When it’s all said and done, I want to be able to say that I created vaginal care and sexual health products that improved women’s health.”
The support and expertise from Mattek helped Good Clean Love, a certified B Corp committed to balancing profit and purpose, diversify and expand its portfolio of personal lubricants, feminine hygiene and vaginal care products. Strgar’s persistence proves that anyone can influence and inspire leaders to change the future of health. “I feel proud that we’ve taken these steps with the FDA on behalf of better women’s health products,” Strgar says. “When it’s all said and done, I want to be able to say that I created vaginal care and sexual health products that improved women’s health.”
References
Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a three-dimensional vaginal epithelium model. Toxicology Reports. 2018; 5: 134-140. DOI: 10.1016/j.toxrep.2017.12.011.
Costin GE, Hill E, Brown J, Clippinger AJ. Qualification of a non-animal vaginal irritation method admitted as nonclinical assessment model (NAM) in the Incubator Phase of the United States Food and Drug Administration (US FDA) Medical Devices Development Tool (MDDT). Toxicology in Vitro. 2020; 6: DOI:10.1016/j.tiv.2019.104680.
Author
Kate Robertson, Customer Experience, BICO
Email: kr@bico.com


 “There are currently so many problems with women’s healthcare products,” says Good Clean Love’s CEO Wendy Strgar, who founded the line of organic personal
“There are currently so many problems with women’s healthcare products,” says Good Clean Love’s CEO Wendy Strgar, who founded the line of organic personal