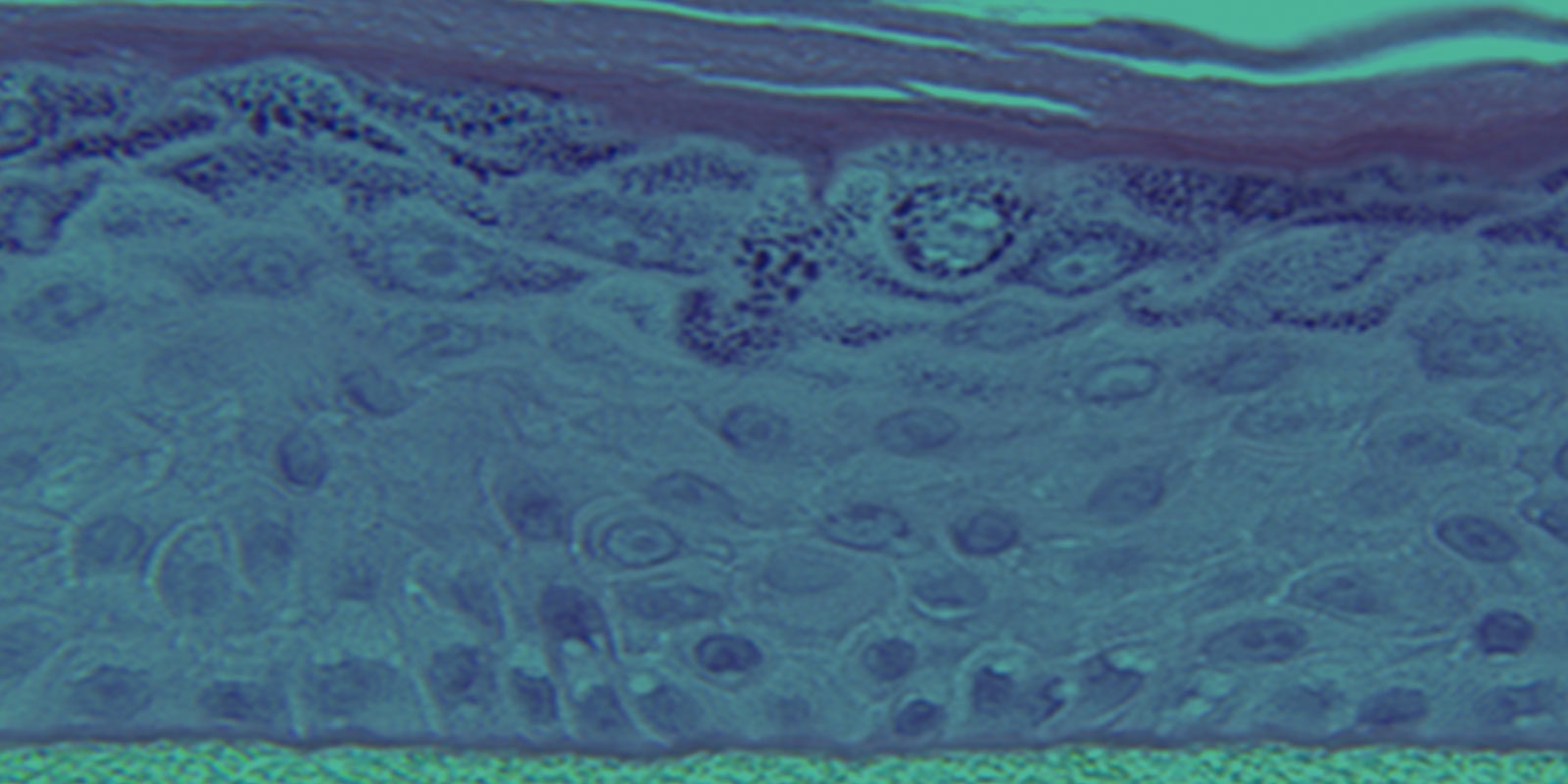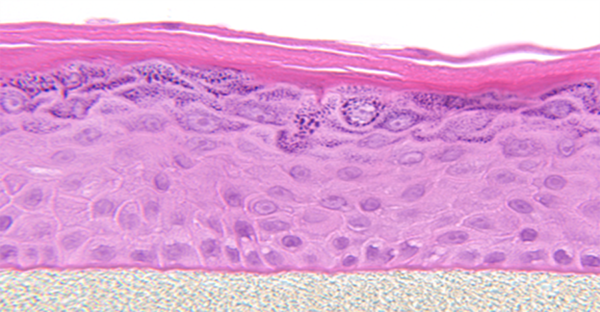
Also known generically as a Reconstructed Human Epidermis (RHE), EpiDerm is a ready-to-use, highly differentiated 3D tissue model consisting of normal, human-derived epidermal keratinocytes (NHEK) cultured on specially prepared tissue culture inserts.

Cultured at the air-liquid interface (ALI), EpiDerm allows for the evaluation of topically applied compounds, chemicals, cosmetic/personal care product ingredients and final formulations. With our straightforward protocols and 20+ years of data, EpiDerm is the gold standard for a broad range of highly predictive in vitro applications.
EpiDerm exhibits human epidermal tissue structure and cellular morphology with greater uniformity and reproducibility. It’s 3D structure consisting of organized and proliferative basal cells, spinous and granular layers, and cornified epidermal layers are mitotically and metabolically active.
The EpiDerm 3D human tissue model is used across a diverse range of applications including safety and risk assessment, and biological efficacy. With Air-liquid Interface (ALI) culture technology, EpiDerm can be used to evaluate biological responses to topical applications of formulations or ingredients. Simple protocols and the evaluation of early cellular endpoints allow research to acquire data in days, not weeks or months.
Skin Irritation (OECD TG 439)
Use EpiDerm to determine the skin irritation potential of liquids, solids, gels, lotions, ointments or creams. Tissue viability results are acquired within 3 days.
Skin Corrosion (OECD TG 431)
Determine the skin corrosion potential of test materials in a single day.
Skin Hydration
Measure the electrical impedance of EpiDerm to assess the efficacy of topically applied moisturizers including creams, gels and lotions. The Skin Hydration protocol allows for data analysis within 24 hours.
Skin Hydration Application Note
Dermal Drug Delivery
Utilize existing transdermal permeation equipment or MatTek’s simple single insert permeation devices to assess API permeation and flux. Determine relative safety with the EpiDerm MTT ET-50 assay.
Percutaneous Absorption Protocol
Phototoxicity
Identify phototoxic effects of topical or systemic test substances using the EpiDerm Phototoxicity Assay (pre-validated)
Dermal Genotoxicity
Identify genotoxic effects of topical or systemic test substances using EpiDerm Genotoxicity Assays (pre-validated)
Comet Assay Protocol
Epidermal Differentiation
Evaluate active compounds for effects on epidermal differentiation using the under-developed EpiDerm tissue model
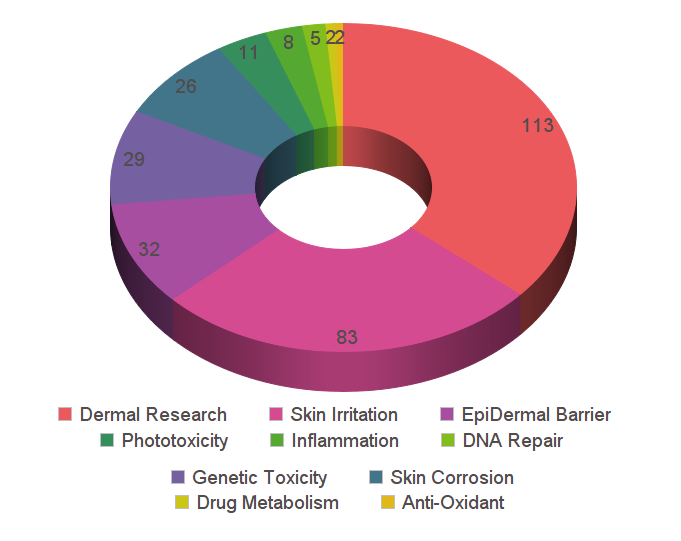
Browse our reference library to see how our EpiDerm tissue has been used in these areas of study.
Tissue
Kit: A standard EpiDerm kit (EPI-200) consists of 24 tissues. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents)
Formats: 9mm & 22mm individual inserts and 96-well HTS plates – tissue culture substrate is chemically modifies with a pore size of 0.4 μm
Culture: Air-liquid interface
Histology: 8-12 cell layers plus stratum corneum (basal, spinous, and granular layers)
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels
Shipment day: Every Monday. Shipment on Thursday also possible upon special request
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 6 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning)
Length of experiments: Cultures can be continued for up to 3 weeks with good retention of normal epidermal morphology. Cultures must be fed every other day with 5.0 ml of long life maintenance medium (EPI-100-LLMM), standard maintenance medium (EPI-100-MM), or new maintenance medium (EPI-100-NMM). Extended culture experiments require the use of culture stands (MEL-STND; see photo) or washers (EPI-WSHR) in 6-well plates to allow for culturing in 5.0 ml of culture medium
Cells
Type: Normal human epidermal keratinocytes (NHEK)
Genetic make-up: Single donor
Derived from: Neonatal-foreskin tissue (NHEK)
Alternatives: NHEK from adult breast skin
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma
Medium
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM)
Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation
Serum: None
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level)
Anti-fungal agent: Amphotericin B 0.25 µg/ml
pH Indicator: Phenol red
Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary)
Alternatives: Phenol red-free (EPI-200-PRF), antibiotic-free (EPI-200-ABF), anti-fungal-free (EPI-200-AFF), or hydrocortisone-free medium and tissue (EPI-200-HCF) are available. Agents are removed at least 3 days prior to shipment. Please discuss your specific media formulation and tissue needs with MatTek scientific personnel before placing your order.
Assay/Maintenance medium: Three maintenance media are available. EPI-100-MM is equivalent to the assay medium used for short term toxicological testing. Long Life Maintenance Medium, EPI-100-LLMM, contains α-MSH and β-FGF. New Maintenance Medium, EPI-100-NMM, contains Keratinocyte Growth Factor (KGF), but does not contain α-MSH and β-FGF. Although all media will result in good retention of epidermal morphology for up to 3 weeks (5.0 ml changed every other day), use of EPI-100-NMM will give the optimal histology for long term studies.
Quality Control and Sterility
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment
End-use testing: Tissues are exposed to 1% Triton X-100 for 4, 6, 8 and 12.5 hours. The time of exposure required to reduce the tissue viability (ET-50) using the MTT viability assay is determined (See MatTek EpiDerm MTT ET-50 Protocol) for each lot of tissue. ET-50’s must fall within the range of the 1996 EpiDerm database of 4.77 – 8.72 hours. ET-50’s in customers’ labs may differ slightly from the MatTek results.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment)
New Orders
To ensure that the most appropriate products and accessories for your application are included with your initial order, MatTek strongly recommends a complimentary consultation with one of our highly trained Technical Specialists. Please contact Viktor Karetsky in the U.S., or Dr. Helena Kandarova in Europe, to order EpiDerm products.
Viktor Karetsky
MatTek Corporation
vkaretsky@mattek.com
T: (508) 881-6771 Ext. 337
F: (508) 879-1532
Helena Kandarova, Ph.D.
MatTek In Vitro Life Science Laboratories
Bratislava, Slovak Republic
hkandarova@mattek.com
T: +421-2-3260-7401
All Other Orders
Please contact Customer Service
Customer Service
MatTek Corporation
T: +1 (508) 881-6771
F: +1 (508)-879-1532
information@mattek.com