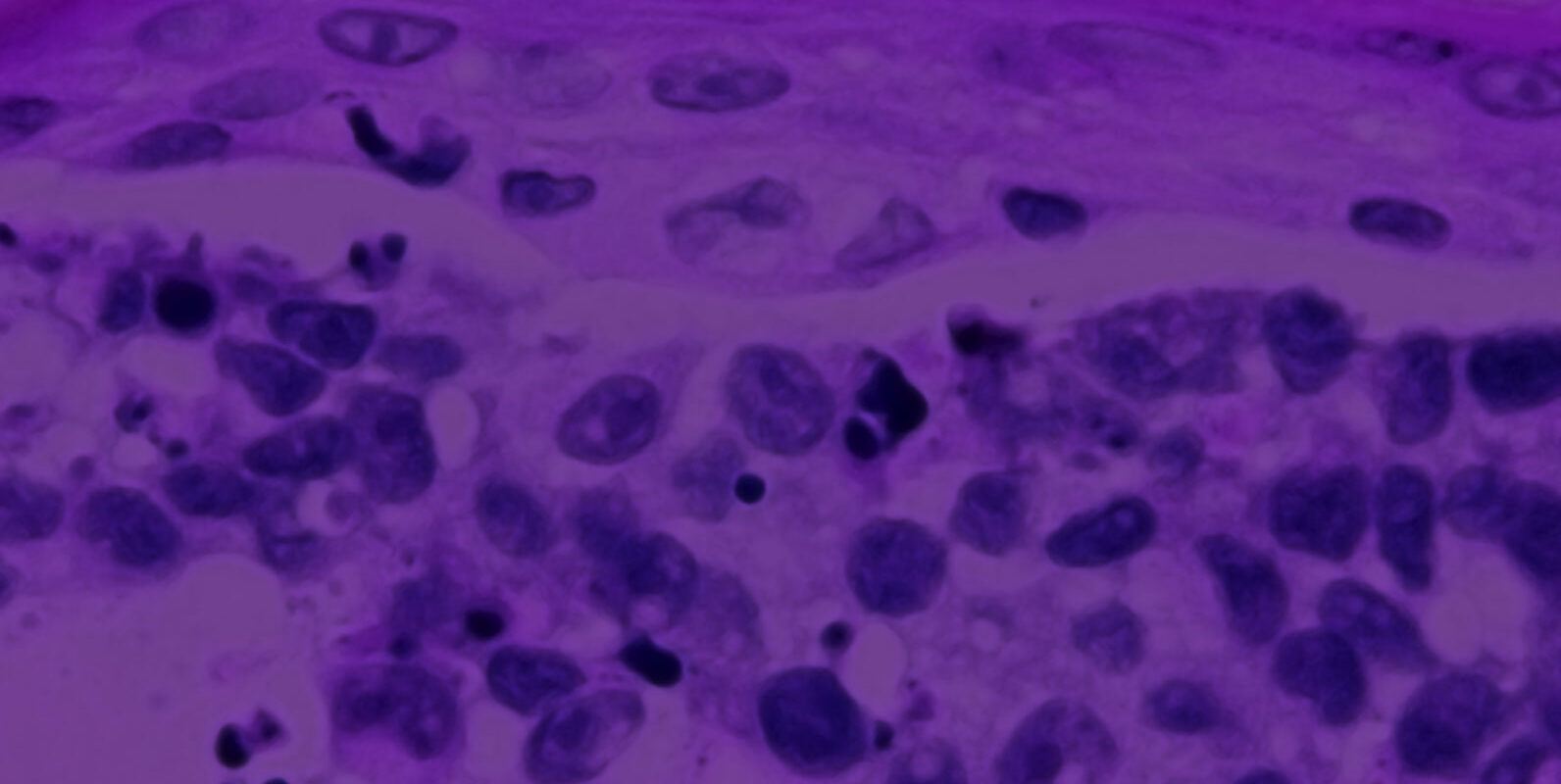
The Melanoma model consists of human malignant melanoma cells (A375), normal, human-derived epidermal keratinocytes (NHEK) and normal, human-derived dermal fibroblasts (NHDF) which have been cultured to form a multilayered, highly differentiated epidermis with melanoma cells at various stages of CM malignancy. At different stages of the culture, the tissue exhibits radial growth phase (RGP), vertical growth phase (VGP), or metastatic melanoma phenotype. The cells are cultured on cell culture inserts using serum free medium, and attain levels of differentiation on the cutting edge of in vitro skin technology. Structurally, the Melanoma model closely parallels the progression of melanoma in vivo, thus providing a valuable tool to study, understand, and develop preventative and therapeutic treatments for one of the most serious cutaneous malignancies.
The Melanoma model exhibits in vivo-like morphological and growth characteristics which are uniform and highly reproducible. Epidermis of this full thickness skin model consists of organized basal, spinous, granular, and cornified epidermal layers analogous to those found in vivo. The dermal compartment is composed of a collagen matrix containing viable normal human dermal fibroblasts (NHDF).
The protocols for using the Melanoma tissues are clear and straightforward. MatTek’s Melanoma tissues have been utilized with a number of target and anti-melanoma drugs.
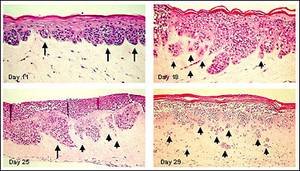
Figure 1. Human Metastatic Melanoma Cells (A375) in Full Thickness Melanoma Skin Model. A375 cells develop RGP melanoma nodes at dermal/epidermal junction (Day 11). With extended culture time, melanoma nodes adopt a VGP morphology (Day 18) and subsequently isolated clusters of cells invade the dermis (metastatic invasion) (Day 29). Long arrows indicate melanoma cell clusters at the epidermal-dermal junction. Short arrows show separated melanoma cell clusters infiltrating the dermis.
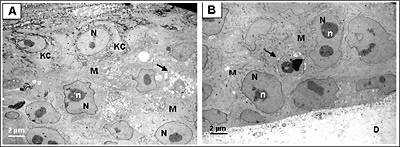
Figure 2. Ultrastructural analysis of Melanoma FT Skin Model.
Transmission electron micrograph (TEM) of the full thickness skin melanoma model (MLNM-FT-A375). A. Area of interaction of melanoma cells (M) and keratinocytes (KC). B. Area of interaction of melanoma cells and the underlying dermal substrate (D). N, nucleus, n, nucleoli. Arrow indicates apoptotic melanoma cells
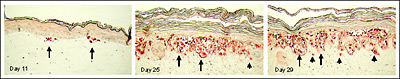
Figure 3. Full thickness skin melanoma model (MLNM-FT) containing metastatic SK-Mel-28 cells. S-100 antibody staining. Initially small nests of melanoma cells form at the dermal/epidermal junction (Day 11). Later VGP tumors develop (Day 25), and subsequently metastasis into the dermis is observed (Day 29). Long arrows indicate some of the melanoma cell clusters at the epidermal-dermal junction. Short arrows show individual melanoma cells infiltrating the dermis.
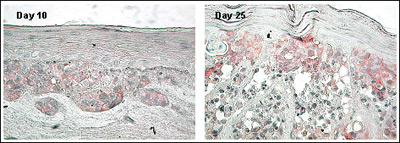
Figure 4. Expression of N-Cadherin Adhesion Molecule in MLNM-FT-A375 Full Thickness Melanoma Skin Model. N-Cadherin antibody staining, 40X. Note increased level of N-Cadherin expression with time in culture.
Tumor Invasion and Anti-Melanoma Drug Screening
MatTek’s Melanoma tissue exhibits radial growth phase, vertical growth phase, and metastatic melanoma phenotypes, paralleling the progression of melanoma in vivo. This model provides a valuable tool for oncologists and cancer researchers to study, understand, and develop preventative and therapeutic treatments for one of the most serious cutaneous malignancies. Browse our Melanoma references in the MatTek Reference Library to see how doctors and researchers have used our Melanoma tissue.
Tissue:
Kit: Full thickness melanoma skin construct (MLNM-FT-A375 kit) consists of 24 tissues. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents.)
Substrate: Single well tissue culture plate inserts are used (e.g. Millicell CM single well tissue culture plate inserts, pore size = 0.4 µm, surface area = 0.6 cm2).
Culture: Initially submerged and then at the air liquid interface.
Histology: Epithelium: 8-12 epithelial cell layers plus stratum corneum (basal, spinous, and granular layers); Dermis: Collagen gel containing fibroblasts. At early stage melanoma cells form nodes at dermal/epidermal junction. At later stages melanomas progress to RGP (radial growth phase), VGP (vertical growth phase) and consecutively invade dermal component.
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: Tissues are shipped at 4°C on medium-supplemented, agarose gels in 6-well plates.
Shipment day: Monday. Shipment on Thursday also possible upon special request.
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 3 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
Length of experiments: Cultures can be continued for up to 4 weeks for melanoma development and progression with good retention of normal epidermal morphology. Cultures must be fed every other day using MatTek culture stands (MEL-STND; click on this link for photo) along with 5.0 ml of MLNM-FT-MM.
Alternative tissues:
MLNM-FT-A375 (Day 6): Early stage MLNM-FT-A375 tissue. Tissue has not been cultured at the Air-Liquid Interface. Customer receives required media (MLNM-FT-GM) to continue cultures and produce MLNM-FT-A375. Discussion with a MatTek technical representative is required prior to ordering due to proprietary nature of this product. Designed for study of early stages of melanoma development in which experimental design dictates use of early stage tissue.
MLNM-FT-EXP: Same as MLMN-FT-A375 except MatTek replaces A375 cells with customer-supplied melanoma cells.
Cells:
Type: Human melanoma cells (A375); Normal human epidermal keratinocytes (NHEK); Normal human dermal fibroblasts (NHDF).
Derived from: Malignant melanoma cell line (A375); Neonatal-foreskin tissue (NHEK); Neonatal skin (NHDF);
Alternatives: Human malignant melanoma cells SK-Mel-28.
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma.
Medium:
Base medium: MCDB 153 Basal Medium.
Growth factors/hormones: Epidermal growth factor, insulin, hydrocortisone and other proprietary stimulators of epidermal differentiation.
Serum: None.
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level).
Anti-fungal agent: Amphotericin B 0.25 µg/ml.
pH Indicator: Phenol red (0.0012 g/l).
Other additives: Lipid precursors used to enhance epidermal barrier formation (proprietary).
Assay/Maintenance medium: MLNM-FT-MM is utilized for assays as well as long term maintenance of the MLNM-FT-A375 tissues.
Quality Control and Sterility:
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
End-use testing: Tissues from the production lot are retained, grown for additional 7 days, fixed for histology, and analyzed.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances sterility failures will be notified within 8 days of shipment).
New Orders
To ensure that the most appropriate products and accessories for your application are included with your initial order, MatTek strongly recommends a complimentary consultation with one of our highly trained Technical Specialists. Please contact Jonathan OIdach to order Melanoma products.
Jonathan Oldach
MatTek Corporation
joldach@mattek.com
T: (508) 881-6771 Ext. 210
F: (508) 879-1532
All Other Orders
Please contact Customer Service via telephone or fax.
Customer Service
MatTek Corporation
T: (508) 881-6771
F: (508)-879-1532
information@mattek.com