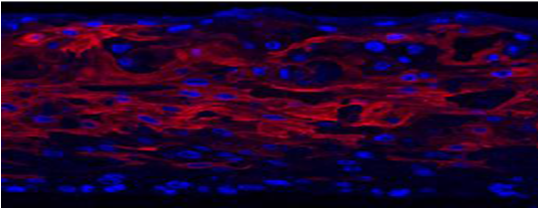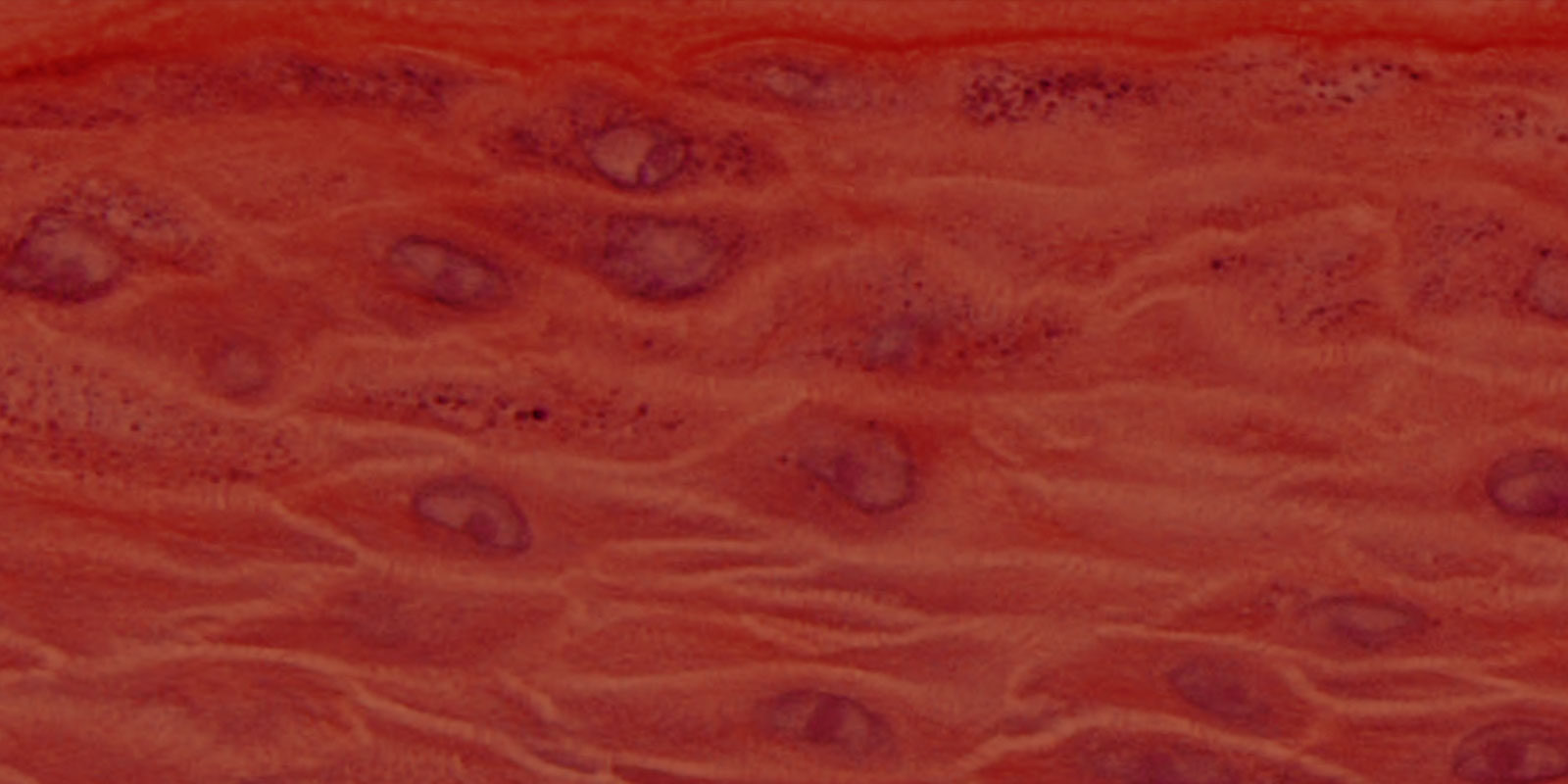
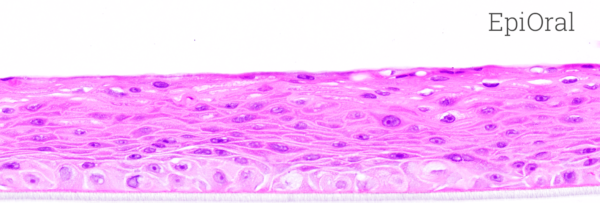
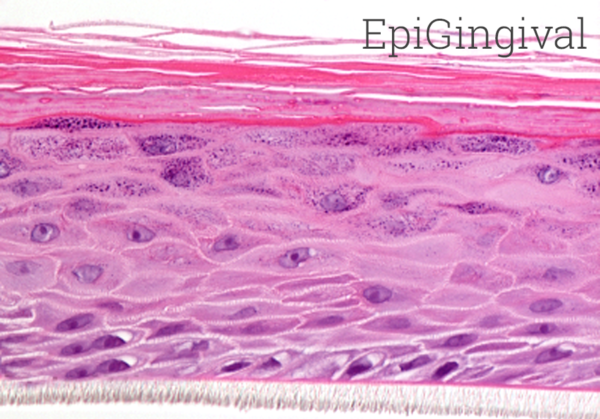
MatTek’s EpiOral and EpiGingival tissues consist of normal, human-derived oral epithelial cells. The cells have been cultured to form multilayered, highly differentiated models of the human buccal (EpiOral) and gingival (EpiGingival) phenotypes. The tissues are cultured on specially prepared cell culture inserts using serum free medium and attain levels of differentiation on the cutting edge of in vitro cell culture technology. The EpiOral and EpiGingival tissue models exhibit in vivo-like morphological and growth characteristics which are uniform and highly reproducible.
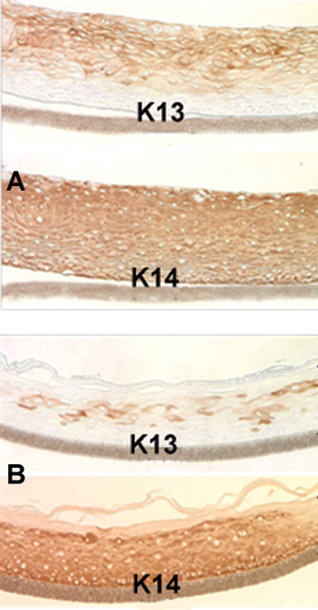
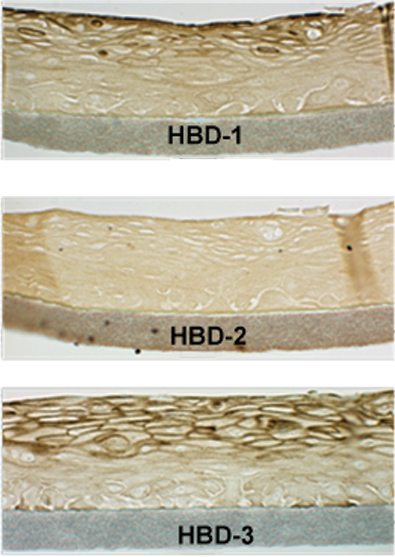
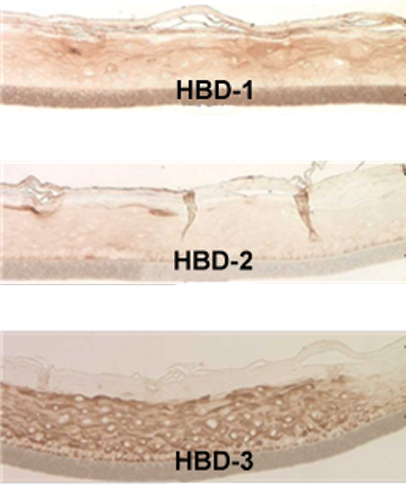
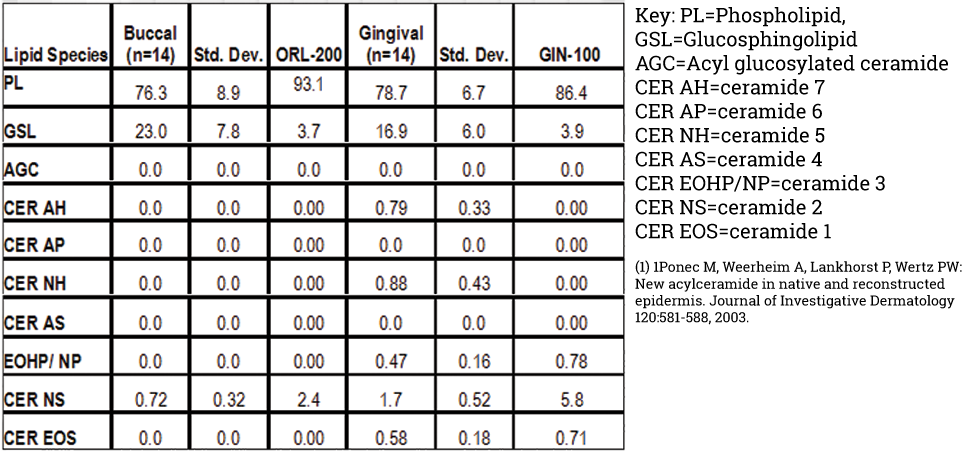
Both EpiOral and EpiGingival express cytokeratin K13 in all layers except the most apical ones and weakly express cytokeratin K14 in the upper layers of the tissue (Figure 1). The tissues also produce the naturally occurring antimicrobial peptides called human beta defensins (HBDs). EpiOral constitutively expresses HBD-1 and HBD-3 but not HBD-2 (Figure 2). EpiGingival weakly expresses HBD-3 in all layers except the uppermost ones and HBD-1 in the apical layers but does not express HBD-2 (Figure 3).
Lipid analysis of the explant tissues along with EpiGingival (GIN-100) and EpiOral (ORL-200) is shown below in Table 1. EpiOral and buccal explants contain only Ceramide 2 (Ceramide NS). EpiGingival and gingival tissue contain Ceramides 1, 2, and 3 (also referred to as Ceramides EOS, NS, and EOHP/NP, respectively).
A growing body of data indicates that EpiOral and EpiGingival effectively provide an inexpensive, non-animal means to assess oral irritation, toxicology, and pathology-related issues. A representative dose-response curve for the effect of sodium dodecyl sulfate (SDS), a common surfactant used in toothpaste, is shown in Figure 4.
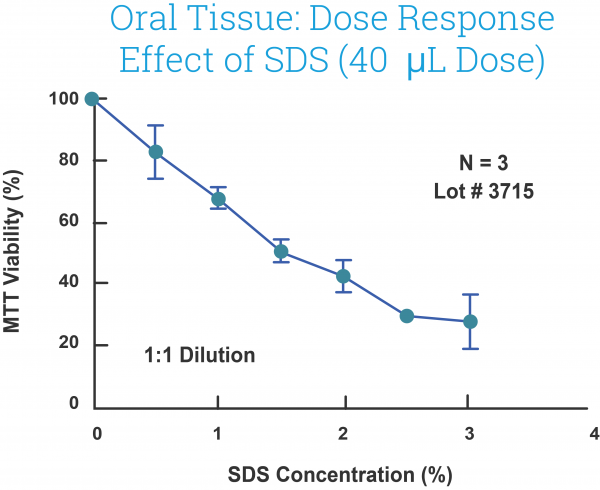
Figure 4: Effect of SDS solutions (40L) on EpiOral (ORL-200) tissue viability following exposure for 1 hour. SDS concentrations were chosen to be in range normally present in toothpaste (0.0 – 3.0%).
Table 1: Lipid analysis of in vivo and in vitro tissues. The in vivo buccal and gingival values are averages for 14 tissue samples. The in vitro tissue values are from a single lot.
Mucosal and Gingival Irritation
Measure oral or gingival epithelial tissue viability and cytokine release using a straightforward MTT assay or commercially available ELISA kit, and obtain definitive results in a few days.
EpiOral & EpiGingival Irritation Protocol
Drug Delivery
EpiOral and EpiGingival are useful in determining the absorption potential of final formulations in vitro.
EpiGingival Drug Absorption Protocol
Model for Oral Pathologies
EpiOral has been used to study oral candidiasis, oral mucositis, and the anti-microbial properties of the oral musosa.
Oral Anti-Microbial Peptides Publication
Search our reference library for more information.
Tissue:
Kits: EpiOral (ORL-200) and EpiGingival (GIN-100) kits consist of 24 tissues. ORL-606 and GIN-606 kits consist of 6 tissues. Tissue kits contain tissues, culture medium, to equilibrate tissues and culture them for 24 hours, and plasticware. Contact MatTek for specific kit contents.
Substrate: ORL-200 and GIN-100: Collagen coated, 9 mm ID single well tissue culture inserts are used. Pore size = 0.4 µm, Inner Diameter = 0.875 cm. Surface area = 0.6 cm2. ORL-606 and GIN-606: Single well tissue culture inserts. Pore size = 0.4 µm, Inner Diameter = 2.5 cm. Surface area = 4.2 cm2.
Culture: At air-liquid interface.
Histology: ORL-200: 8-11 cell layers of non-cornified tissue. GIN-100: 8-11 layers of cornified tissue.
Lot Numbers: Tissue lots produced weekly are assigned a specific lot number. All tissue kits within a lot are identical with regard to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium supplemented, agarose gels.
Shipment day: Monday.
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location.
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 96 hours prior to use. However, extended storage periods are not recommended unless absolutely necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Wednesday morning following overnight storage at 4°C.
Length of experiments: Cultures can be continued for at least 1 week with good retention of normal morphology. Cultures must be fed every other day with 5.0 ml of maintenance medium (ORL-200-MM or GIN-100-MM). Cell culture inserts are placed atop culture stands (MEL-STND) or washers (EPI-WSHR) in 6-well plates to allow the use of 5.0 ml.
Alternative tissues:
ORL-300-FT: Full thickness ORL-200. Oral epithelial tissue cultured on top of a collagen matrix containing gingival fibroblasts.
ORL-300-FT-LC: ORL-300-FT containing Langerhans Cells.
GIN-300-FT: Full thickness GIN-100. Gingival epithelial tissue cultured on top of a collagen matrix containing gingival fibroblasts.
Cells:
EpiOral
Type: Normal human oral keratinocytes (NHOK) are differentiated into tissues with a non-cornified, buccal phenotype, part number ORL-200.
Genetic make-up: Single donor.
Derived from: Non-diseased, adult human oral tissue obtained from patients undergoing tooth extractions or from cadavers. Tissues are produced using cells derived from non-smokers who have no history of drug use or oral pathology.
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma, bacteria, yeast, fungi.EpiGingival
Alternate donors available. EpiOral produced using cells derived from patients with a history of smoking are available (ORL-200-D2).
EpiGingival
Type: Normal human oral keratinocytes (NHOK) are differentiated into tissues with a cornified, gingival phenotype, part number GIN-100.
Genetic make-up: Single donor.
Derived from:
Non-diseased, adult human oral tissue obtained from patients undergoing tooth extractions or from cadavers. Standard tissues are produced from cells derived from non-smokers who have no history of drug use or oral pathology.
Alternate donors available. EpiGingival produced using cells derived from patients with a history of smoking are available (GIN-100-D2).
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma, bacteria, yeast, fungi.
Medium:
EpiOral &EpiGingival
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM)
Growth factors/hormones: Epidermal growth factor and other proprietary factors
Serum: None
Antibiotics: Gentamicin 5 µg/ml
Anti-fungal agent: Amphotericin B 0.25 µg/ml
pH Indicator: Phenol red
Other additives: Proprietary
Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free medium and tissue are available. Agents are removed 3 days prior to shipment
Maintenance medium: ORL-200-MM or GIN-100-MM
Quality Control and Sterility:
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment.
End-use testing:. Tissues are also exposed to 1.0% Triton X-100 for various times and the exposure time required to reduce the tissue viability to 50% (ET-50) is determined using the MTT assay. QC specifications: ORL-200: 34.85 < ET-50 < 105.78 min GIN-100: 5.47 hr < ET-50 < 10.42 hr.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination.
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service.
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because some of our QC and sterility testing is done post-shipment, notification will be made as soon as possible. Under normal circumstances, ET-50 failures will be notified by Thursday, 5 p.m.; sterility failures will be notified within 8 days of shipment.
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**