In Vitro Irritation Assay and Regulatory Acceptance Two Years after ISO 10993-23:2021 Release
- TR Number: 1015
- Authors: C. D. Christensen, S. M. Street, S. B. Pascua, K. P. Coleman, and W. V. Christian
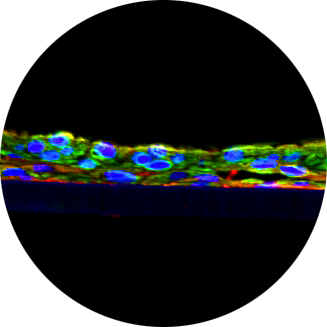
Medical device biological safety testing is transitioning away from the use of animals to more technologically advanced and reliable in vitro alternative methods. The irritation potential of medical devices has historically been evaluated by rabbit testing; however, ISO 10993-23:2021 offers a validated alternative by using reconstructed human epidermis (RhE) as the first consideration. Regulatory agencies and notified bodies, such as U.S. FDA and TÜV SÜD, have placed greater emphasis on reducing the use of animals in medical device testing, but there has been inconsistent regulatory acceptance of the RhE in vitro assay two years after ISO 10993-23:2021 was published. With the inconsistent acceptance, manufacturers are often tasked with conducting both the in vitro and in vivo irritation assays to meet the requirements of regulatory bodies across the globe. Recently, regulatory agencies have asked for additional information on the applicability of the in vitro assay regarding topics such as mild irritants, mixtures, and information on incompatible materials. In 2022, we examined 15 paired in vitro and in vivo irritation data sets to understand if the in vitro assay was suitable for use with different types of final, finished medical devices (“Correlation Of In Vitro And In Vivo Skin Irritation Assays In Medical Devices: A Case Study,” SOT Abstract 3474, 2022). This work showed that the in vitro assay produced equivalent results 100% of the time for medical device extracts, which are mixtures. However, a limitation of this study was that a positive result was not obtained in either assay for comparison. Due to the ongoing inconsistent regulatory acceptance of the in vitro model, we have continued to analyze test results from both methodologies. To date, we have collected more than 100 sets of paired data presented herein, including results from devices known to be positive for irritation in the in vivo assay.