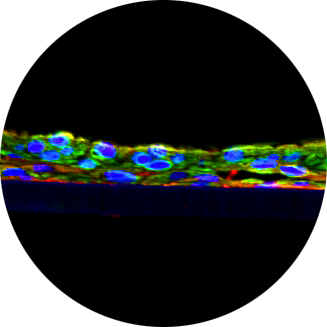
Join Mattek at SOT 2022

 Mattek scientists will be attending and presenting posters at the Society of Toxicology Annual Meeting in San Diego, California. Read more to see what they’ve been working on and request copies of their posters. We can’t wait to see you at SOT 2022!
Mattek scientists will be attending and presenting posters at the Society of Toxicology Annual Meeting in San Diego, California. Read more to see what they’ve been working on and request copies of their posters. We can’t wait to see you at SOT 2022!
Join the Bio-Convergence Revolution 
Meet our team at Booth #1217
We’d love to chat: Schedule a Meeting
Poster Presentations:
Primary Human Cell-Based 3D Colon Tissue Model for Toxicological Studies
Jon Oldach, Camden Holm, Yulia Kaluzhny, Alex Armento, Seyoum Ayehunie.
Mattek Corporation, 200 Homer Avenue, Ashland, MA 01721.
Session:
Wednesday, March 30 | 10:45AM – 12:30PM
Abstract
The aim of this study was to reconstruct and characterize a primary cell based human 3D colon tissue model for the study of 1) microbiome, 2) colorectal care product screening for their irritation potential, 3) safety and efficacy of anti-microbial products and colon targeted drug candidates, and 4) inflammation. This study characterizes the structural features of a novel in vitro tissue model reconstructed from normal human primary colon epithelial cells (CEC) with or without fibroblasts. Primary cells were expanded in monolayer culture and seeded onto microporous membrane inserts to reconstruct 3D organotypic CEC tissues. Then, tissues were characterized for polarity of epithelial cells (H&E staining), and fibroblast cell markers (confocal microscopy), barrier integrity (transepithelial electrical resistance, TEER) measurement, and its functionality for toxicological studies was tested using Indomethacin (0.01-0.5mg/mL) and SN38 (20 uM) following acute apical exposure for 24 hr. Analysis of the 3D colon tissue model revealed: 1) wall-to-wall tissue growth, 2) epithelial cell morphology similar to human colon, 3) a physiological TEER value of >300 Ώ*cm2 mimicking the colon microenvironment, and 4) expression of CK19 (epithelial cell marker), vimentin (fibroblast cell marker), and Alcian blue PAS staining (mucous producing goblet cell marker). Acute exposure to indomethacin (0.5%) and SN38 (20uM) showed a 21.2% and a 22.3% decrease in TEER values, respectively, compared to untreated controls which indicates toxicity of the test articles. This novel human cell-based CEC tissue model will be a useful tool for pre-clinical assessment of microbiomes, mucosal inflammation, and screening of colorectal care products for their irritation potential. Such models can also reduce the use of animals for experimentation.
Organotypic 3D primary human nasal tissue model for toxicological studies.
Seyoum Ayehunie¹, Zachary Stevens¹, Julia Oh², Yulia Kaluzhny¹, Alex Armento¹.
¹Mattek Corporation, 200 Homer Avenue, Ashland, MA.
²The Jackson Laboratory, 10 Discovery Drive, Farmington, CT 860-837-2014
Session:
Monday, March 28 | 9:00AM – 10:45AM
Abstract
The nasal mucosa serves as the first line of defense against inhaled chemicals, drugs, and respiratory infections. Here we developed a novel in vitro primary human cell-based 3D human nasal epithelial tissue (NET) on a transwell porous membrane at an air-liquid interface (ALI). The NET tissue was characterized by histology, barrier function (TEER), viability (MTT), toxicity, and infection with viruses. Histological and immunohistochemical evaluation of the in vitro nasal model showed polarized and multilayered tissue that expresses epithelial cell marker (CK19), tight junction (ZO-1 and Claudin-1), mucin (MUC5B and MUC5AC), SARS-CoV-2 entry-related /proteins/genes (ACE-2 and TMPRSS2), and cilia (alpha-tubulin). Single cell sequencing of the differentiated NET cultures also confirms the presence of differentiated cell types (goblet, club cells, basal, multiciliated cells, and not previously identified cells (NPIC)). The NPIC were enriched in response to allergic rhinitis/respiratory syncytial virus infection (increase in IL1RL1, MMP9, MMP10) and some laminins and integrins. Infection of NET with viruses also showed high expression IDO1, which is associated with innate antiviral immune functions. To monitor reproducibility, the MTT and TEER results from N=9 lots were analyzed. MTT showed OD value of >1.0 (mean OD =1.52±0.23). The mean TEER value for the N=9 lots was 303±98 Ω*cm2. To evaluate the utility of the nasal tissue model for toxicological studies, we tested the effect of a known mucous membrane irritant butylamine following 4 hr topical exposure to 0.5 mg/ml and 2 mg/ml of the test article. The data showed reduction in barrier integrity by 33.2% ± 0.0 and 12.3 ± 5.8, respectively. compared to the vehicle control (corn oil) which is indicative of toxicity of the test article. In short, this novel NET model can be added to the toolbox of 3D respiratory tissue models to predict safety of chemical inhalants, therapeutic candidates, viral and bacterial infections at entry site.
Request a Copy
Application of physiologically relevant in vitro inhalation model to predict acute respiratory toxicity of mists and volatile liquids
Yulia Kaluzhny¹, George R. Jackson¹, J. Marcus², Olivia Gabriel¹, Paul Kearney¹, S. Letasiova², Mitchell Klausner¹, and Alex Armento¹
¹Mattek Corporation, Ashland, MA, USA
²Mattek In Vitro Life Science Laboratories, Bratislava, Slovakia
Session:
Tuesday, March 29 | 2:30PM – 4:15PM
Abstract
Acute respiratory toxicity (ART) testing is required to assess the health effects of inhaled substances. OECD accepted methods utilize GHS categorization that is based on animal death. There is no validated in vitro ART assay, even though animal tests have been discredited as predictors of human responses and on ethical grounds. The goals of this work were to develop physiologically relevant ART tests using the EpiAirway™ tissue model, demonstrate interlaboratory transferability, and correlate the results to an established categorization system relevant to human respiratory irritation.
Test articles (TA) were applied to EpiAirway tissues (0.6 cm2) at Mattek (USA, n=53) and IVLSL (Slovakia, n=43) with ART protocols developed for exposure to mists/sprays (Direct Application Protocol, DAP) and vapors/volatile liquids (Vapor Cap Protocol, VCP). In both protocols, tissues were exposed for 4 hours to 4 fixed doses of the TA (0.5, 2, 10, 20 mg/tissue, diluted in corn oil or water). In the DAP, TAs were applied to the apical tissue surface and in the VCP – to an absorbent material in a specially designed cap that forms a tight seal above the tissue allowing exposure to TA vapor. The effects on tissue viability (MTT assay) and barrier properties (Transepithelial Electrical Resistance, TEER) were determined. The effective doses which reduced tissue viability by 25% (ED-25) or by 75% (ED-75) were mathematically interpolated for the DAP and VCP methods, respectively. The ED-25 and ED-75 were correlated to the acute irritation Health Effects (HE) Codes (HE14/15/16) listed by OSHA, which are relevant to human exposure. Using the MTT assay, the DAP discriminated between HE14/15/16&NH with a Sensitivity/Specificity/Accuracy (S/S/A) of 72.2/85.5/78.8%; correlation to GHS Cat.1&2/3&4/5&NC gave results of 55.9/74.0/64.9% S/S/A. The VCP discriminated between HE codes with S/S/A of 80.9/90.5/85.7% (Mattek) and 77.0/88.7/82.8% (IVLSL); correlation to GHS was 68.0/81.2/74.6% S/S/A (Mattek) and 62.2/76.1/69.1% (IVLSL).
Both protocols demonstrated high predictivity of human HE Codes, which are more relevant to human respiratory toxicity than the GHS categories. Good inter-laboratory reproducibility was observed for the VCP. The VCP and DAP provide physiologically relevant, organ-specific in vitro tests that can improve the predictivity of human responses and significantly reduce the number of animals being used.
Request a Copy
3D Rat Airway Tissue Model for Translational Toxicological Studies
Mateo Frare, Robert Jackson, Samantha Durand, Yulia Kaluzhny, Tim Landry, Alex Armento, and Seyoum Ayehunie.
Mattek Corporation, 200 Homer Avenue, Ashland, MA 01721.
Session:
Monday, March 28 | 9:00AM – 10:45AM
Abstract
A scalable and reproducible in vitro 3-dimensional organotypic model of rat airway tissues that will replicate biological responses is needed to provide an alternative to traditional in vivo rat toxicity tests. The aim of this study was to test the utility of a primary cell-based 3D rat epithelial airway tissue (REAT) model to screen: 1) toxicants, 2) inhaled drugs, and 3) formulations for their irritation potential. To achieve this goal, primary cells were expanded in monolayer culture and seeded onto microporous membrane inserts to reconstruct 3D organotypic REAT tissues. Then, tissues were characterized for polarity of epithelial cells (histology), epithelial cell markers (confocal microscopy), barrier integrity (transepithelial electrical resistance, TEER), and expression of drug transporters and drug metabolizing enzymes (RT-PCR). Functionality for toxicological studies was tested using 4 chemicals of different hazard classifications at four concentrations (5, 25, 125, and 250 mg/mL). Analysis of the 3D REAT revealed a well polarized epithelium and a physiological TEER value of >300 Ώ*cm2 mimicking the airway microenvironment. RT-PCR results showed expression of drug transporters (influx and efflux), drug metabolizing enzymes (aldehyde dehydrogenases 2, 3a1, 3b1, 5a1, 6a1, 7a1, and 9a1), CYP450 enzymes (1b1, 26b1, 2c80, 2f4, 2s1, 2t1, 3a9, and 4f6), and esterase D. Acute exposure to the chemicals showed varying levels of tissue viability (toxicity) by MTT assay. Based on the minimum concentration required to reduce tissue viability by 25%, the test chemicals were rank ordered from high to minimal toxicity: Propamocarb (25 mg/mL) > Diazinon (125 mg/mL)> Malathion (250 mg/mL)> Permethrin (>250 mg/mL), vehicle control. Thus, the REAT tissue model will be a useful tool to predict rat airway toxicity and bridge rodent to human translation of in vitro inhalation toxicology data. Such models can also reduce the use of animals for experimentation.
Request a Copy
New high throughput cell culture plate for 3-dimensional (3D) tissue culture and toxicological testing
Mitchell Klausner, Yulia Kaluzhny, George R. Jackson, Paul Kearney, Alex Armento
Mattek Corporation, Ashland, MA, USA
Session:
Monday, March 28 | 9:00AM – 10:45AM
Abstract
A new, 96-well high throughput (HTP) cell culture insert plate with a microporous filter bottom has been developed for toxicological testing. The microporous polycarbonate membrane bottom has a high pore density (1 x 108 pores/cm2) and an average pore size of 0.4 µm allowing for culture of highly differentiated 3D tissue models. The utility of the new HTP plate was demonstrated by culturing tracheal-bronchial epithelial cells for a 2-week period to form an organotypic model of upper respiratory tissue. H&E stained histological cross-sections of the tissue showed a pseudo-stratified morphology with both ciliated and mucous producing cells, which was similar to the morphology of single-well cell culture insert (SW-CCI) versions of the tissue. The barrier properties of the HTP tissues, as measured by transepithelial electrical resistance (TEER), were not significantly different from those of the SW-CCI tissues. TEER measurements were 654 ± 204 (n=96) and 809 ± 147 (n=4) Ω*cm2, for the SW-CCI and HTP tissues, respectively (p=0.12). The utility of the tissues for toxicological testing was investigated using 3 respiratory irritants (RI), acetone (AC), methyl propyl ketone (MPK), and formaldehyde (FOR), and results for the HTP tissues were compared to results from tissues cultured in SW-CCIs. 4 doses of each RI were applied to the apical side of the tissues for 4 hrs, and tissue viability was determined after a 20-hour recovery period using the MTT assay. The effective dose which reduced tissue viability by 25% (ED-25) was mathematically interpolated. The ED-25 values for the HTP and SW-CCI tissues were comparable. The ED-25 values for HTP tissues were 38.6, 22.0, and <0.8 mg/cm2 of tissues surface for AC, MPK, and FOR, respectively, and for the SW-CCI tissues, ED-25 values were 45.1, 20.9, and <0.8 mg/cm2. We anticipate that this new HTP plate will be useful for toxicological screening of large numbers of formulations during the drug development/ formulation process.
Request a Copy
Identification and Sub-categorization of Ocular Irritants Using the EpiOcular Tissue Model – Prediction Models for Liquids and Solids
Silvia Letasiova1, Lenka Hudecova1, Jan Markus1, Yulia Kaluzhny2, Mitch Klausner2
¹Mattek In Vitro Life Science Laboratories, Mlynske Nivy 73, 82105 Bratislava, Slovakia
²Mattek Corporation, 200 Homer Ave, Ashland, MA, USA
Session:
Monday, March 28 | 9:00AM – 10:45AM
Abstract
Determination of serious eye damage/eye irritation originally involved the use of laboratory animals (OECD TG 405). In 2015, a new test guideline (OECD TG 492) was accepted which enables the use of an in vitro procedure based on reconstructed human cornea-like epithelium (RhCE) to distinguish between chemicals (substances and mixtures) not requiring classification and those that must be labeled for eye irritation or serious eye damage. Chemicals identified as requiring classification for eye irritation/serious eye damage must be further tested to distinguish between eye irritants and those causing serious eye damage. There have been several projects focused on the development of tiered testing strategies for eye irritation assessment which takes in account all drivers of classification. The goal of these projects has been to develop a testing strategy to sub-categorize chemicals which: a) do not require labeling for serious eye damage or eye irritancy (No Category), b) can cause serious eye damage (Category 1 or Cat 1), and c) are eye irritants (Category 2 or Cat 2).
In the current project, a set of 13 chemicals (7 liquids and 6 solids) that are listed as proficiency chemicals in draft OECD TG 492B were tested using the RhCE model, EpiOcular. We used a testing strategy developed in CON4EI project and confirmed in ALT4EI project, which combines the most predictive time-points of EpiOcular time-to-toxicity neat and dilution protocols. Liquids and solids were test separately with different methodologies and prediction models. The set of chemicals consisted of 4 Cat 1 chemicals, 5 Cat 2 chemicals and 4 No Cat chemicals. Using the proposed testing strategy, we were able to correctly identify 100% of Cat 1 chemicals (4/4), 100% of Cat 2 chemicals (5/5) and 100% of No Cat chemicals (4/4).
The testing strategy proposed in CON4EI and verified in ALT4EI projects to achieve optimal prediction for all three categories – prediction models for liquids and solids seems to be a very promising tool in an integrated testing strategy (ITS) that can discriminate chemicals to No Cat, Cat 2 and Cat 1.
Request a Copy
Organotypic small intestinal model for long term high throughput screening toxicity studies
Zachary Stevens, Paul Kearny, Mitch Klausner, Yulia Kaluzhny, Alex Armento, and Seyoum Ayehunie.
Mattek Corporation, 200 Homer Avenue, Ashland, MA 01721.
Session:
Wednesday, March 30 | 10:45AM – 12:30PM
Abstract
The EpiIntestinal tissue model has been used to study a variety of phenomena affecting the gastrointestinal (GI) tract including drug permeation and metabolism, drug toxicity, GI inflammation, and microbial infection. Most recently, the model has been used to investigate infection with SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic. The current work focuses on adapting this model to a newly fabricated 96-well, high throughput format for extended culture period for chronic studies. Normal human small intestinal cells were seeded onto the 96-well, microporous membrane bottom plates and cultured for 2 weeks to reconstruct the intestinal tissues. The resulting tissues have a villi-structure and barrier properties similar to native intestinal tissue, as evidenced by transepithelial electrical resistance (TEER) values of 160-300 Ohm* cm2. These cultures were maintained for over 3 months and tissue histology and barrier integrity were monitored weekly. To monitor well-to-well tissue reproducibility, TEER measurement was performed every week on all 96-well tissues. Histological cross-sections showed that tissues fixed at all time points up to and beyond 3 months contained structures and morphology similar to the standard EpiIntestinal tissue model and native intestinal tissue. Immunohistochemical staining also showed epithelial marker (CK19), tight junction formation (ZO-1), and brush border marker villin similar to the standard tissue model. Availability of intestinal organotypic tissue that can be cultured for extended period of time (3 months) can be used for chronic exposure toxicity studies. In conclusion, the newly fabricated plates support reconstruction of EpiIntestinal tissues for extended time period that can be used for hazard identification of chemicals and nanoparticles affecting the GI tract in a high throughput format and to study drug safety and efficacy following chronic exposure.
Request a Copy
Use of an in vitro reconstructed human corneal tissue model to evaluate ocular side effects of systemic medications
Miriam Kinuthia, Yulia Kaluzhny, Mitch Klausner, Alex Armento
Mattek Corporation, Ashland, MA, USA
Session:
Monday, March 28 | 9:00AM – 10:45AM
Abstract
Chronic use of systemic medications can cause light sensitivity, pain, corneal edema/inflammation, and/or cytotoxicity. Animal tests are often poor predictors of human responses. There is a worldwide need for physiologically relevant, human primary cell-based tissue models to address ocular safety for the evaluation of new drug formulations.
We have utilized an in vitro reconstructed EpiCorneal™ tissue model to analyze the effect of frequently used drugs with known adverse ocular side effects. EpiCorneal tissues are cultured using normal human corneal epithelial cells, express site-specific mucins and tight junctions, and attain morphology, barrier properties (Transepithelial electrical resistance or TEER > 1000±200 Ω*cm2), and gene expression comparable to the in vivo human cornea. Tissue performance, evaluated by TEER and tissue viability (MTT assay), were comparable after 24h and 96h under simulated shipping conditions.
The effects of Chlorpromazine hydrochloride (CPZ), a common psychotropic agent; Hydroxychloroquine sulfate (HCQ), an anti-inflammatory / anti-malaria drug; Alfuzosin hydrochloride (ALF), antihypertensive drug; and Fosamax (Alendronate Sodium, FOS), a common anti-osteoporosis agent, were investigated. Endpoints included MTT, TEER, histology, and LDH and cytokine release. Tissues were incubated in the medium containing physiologically relevant concentrations of the drugs for 24h and 48h. For CPZ-treated tissues, the lowest dose to cause a significant decline in barrier function (67.4%) was 12.5 µM at 24h; 25 µM decreased tissue viability (60.5%) at 48h. For HCQ-treated tissues, a decline in TEER (67.4%) was detected for 18.52 µg/ml at 24h, and in viability (53.6%) for 55.56 µg/ml at 48h. For ALF-treated tissues, major declines in TEER and viability were observed at 500 µg/ml after 24h, while an increase in IL-8 release – at 0.005 µg/ml at 48h. For FOS-treated tissues, a significant TEER decrease (57.8%) was detected at 0.1 µg/ml and in tissue viability (85.7%) at 10 µg/ml at 48h. Treatment-specific changes in tissue morphology and dose response of LDH were also observed.
EpiCorneal tissue model is valuable for evaluating formulations with negligible irritation potential. It is suitable for rapid drug screening, will model systemic and topical drug exposure, improve the predictivity of human responses, be more cost effective and reproducible than animal methods. It will facilitate drug discovery worldwide by allowing screening and optimization of pharmaceuticals prior to clinical studies.
Request a Copy
A novel macrophage containing organotypic Small Intestinal tissue for modeling gut inflammation.
Kevin Causey¹, Seyoum Ayehunie¹, Zachary Stevens¹, Timothy Landry¹, Yulia Kaluzhny¹, Anna Langerveld², Elisabeth Lehigh², Stephanie Wheeler², Alex Armento¹.
¹Mattek Corporation, 200 Homer Avenue, Ashland MA.
²Genemarkers, LLC, 126 E. South Street, Kalamazoo, MI.
Session:
Wednesday, March 30 | 10:45AM – 12:30PM
Abstract
As gate keepers of intestinal immune homeostasis, macrophages play a critical role in inflammation in the gut. In this study, we reconstructed a new macrophage-containing primary cell-based full-thickness small intestinal (SMI+M) tissue model and characterized it for: 1) macrophage incorporation (immunohistochemistry, IHC), 2) barrier properties (TEER), and 3) functionality by measuring inflammatory responses following exposure to ligands for TLR4 (lipopolysaccharide; LPS), NOD-1 (C12-iE-DAP) and NOD-2 (L18-MDP) either individually or synergistically. For identification of inflammatory responses, we utilized Affymetrix GeneChip arrays. Results showed that SMI+M tissues have 3D polarity and their morphology and physiological barrier property mimic that of native in vivo tissues. IHC of the SMI+M tissues showed CD14+ (macrophage marker) cells in the underlying fibroblast layer. Using gene upregulation level of >1.9 fold as a cut-off following ligand stimulation, tissues without macrophages (SMI-M) showed fewer upregulated genes (1400 genes) compared to SMI+M (4400 genes). Furthermore, when gene upregulation levels by ligand-induced SMI+M were compared against stimulated SMI-M even higher differences in upregulated genes (> 5200 genes) were noted. In SMI+M, upregulated genes include chemokines, chemokine receptors, FC receptors, co-stimulatory molecules, interferons, and HLA’s which are characteristic of immune cells. When we looked at 13 upregulated genes (>6-fold) in SMI+M, the synergistic effect of ligands in inducing inflammatory gene upregulation was much more pronounced compared to stimulated SMI-M tissues. Furthermore, increased cytokine release of IL-6, IL-8, and TNF-α into the culture medium from ligand exposed SMI+M was also confirmed by BioPlex ELISA. In summary, our results demonstrate that the 3D SMI+M tissue model can serve as an in vitro tool to study the complex cellular interactions manifested during inflammation in the gut microenvironment.
Request a Copy